Progressive Recanalization of a Giant, Partially Thrombosed Basilar Aneurysm despite Sequential Treatment with Pipeline Embolization Device Keywords: aneurysm, embolization, stent, stroke, angioplastyInteractive ManuscriptAsk Questions of this Manuscript: What is the background behind this topic?The Pipeline embolization device has shown great initial promise in the treatment of complex intracranial aneurysms. As clinical experience grows, complications associated with Pipeline embolization are increasingly recognized and reported. Describe your observation(s).In this report, we describe what is to our knowledge the first case of progressive recanalization of an aneurysm in the vertebrobasilar system that occurred despite sequential telescoping placement of several Pipeline embolization devices across the neck of the aneurysm. Describe the major lesson provided by your observation.Progressive aneurysm recanalization can occur despite serial telescoping placement of Pipeline embolization devices (PEDs). Anticoagulation status, perforator-rich zone, and size mismatch between stents were the postulated mechanisms for recanalization in our case. Until the technology is fully mature, we must continue to report its complications in a critical and timely fashion. In response to reviewer 5, we have never seen progressive recanalization of any other intracranial aneurysm despite serial PED placement apart from the present case. What is the background of your topic ?Flow diverters are the latest tool in the armamentarium in the treatment of intracranial aneurysms.  Flow diversion is based on the concept of endoluminal reconstruction accomplished by redirecting flow away from the aneurysm through the parent vessel, leading to progressive aneurysm thrombosis and neointimal growth across the aneurysm neck.  Initial results with the use of these devices were extremely encouraging (6-8)  ; and in April 2011, the FDA approved the Pipeline embolization device (PED, ev3/Covidien, Irvine, California, USA) , a flow diverter, for treatment of large or giant wide-necked intracranial aneurysms from the petrous to superior hypophyseal internal carotid artery (ICA) segments (current on-label indications) (9). Experience with the PED in the treatment of aneurysms in the vertebrobasilar system, however, remains very limited. Describe your main observation and why it is important.As clinical experience accumulates, complications associated with PED, both thromboembolic and hemorrhagic, are increasingly recognized (4, 10). We report a case of progressive recanalization of a giant, partially thrombosed basilar artery aneurysm despite serial treatment with PEDs. An 82-year-old woman, who was otherwise healthy, presented initially with gait ataxia. A magnetic resonance (MR) imaging scan and subsequent cerebral angiogram revealed a giant, partially thrombosed midbasilar aneurysm (2.5 x 2.4 cm) with significant brainstem compression (Fig. 1A,B)  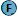 . Because of the patient’s age, the amount of mass effect on the brainstem, and the large amount of intra-aneurysmal thrombus demonstrated on MR imaging, Pipeline embolization was thought to be a better treatment option than stent-assisted coil embolization. With stent-assisted coil embolization, we were concerned of thrombus release during coil embolization, a lack of scaffold for the coils due to the large amount of thrombus, and the potential of exacerbating the existing mass effect. After a thorough discussion of therapeutic options and associated risks and benefits with the patient and her family, the decision was made to proceed with PED reconstruction. The patient received loading doses of aspirin and clopidogrel, with therapeutic responses obtained (aspirin response unit of 426 and 50% inhibition on the Plavix assay). A single 4.25 x 18 mm PED was successfully deployed in the basilar artery across the neck of the aneurysm. The patient did well with no new neurologic deficits. However, her hospital course was complicated by thrombocytopenia (platelet count of 60-70 k/ul) (heparin-induced thrombocytopenia panel was negative and the thrombocytopenia was likely antiplatelet-related) and small bilateral pulmonary emboli (asymptomatic) discovered incidentally on follow-up computed tomographic angiogram (CTA) of the aneurysm, which was completely occluded. She was placed on warfarin for pulmonary embolism. Given the patient’s age and her thrombocytopenia, we decided to discontinue the Plavix once she was therapeutic on warfarin. She was subsequently discharged to a rehabilitation facility on aspirin and warfarin. We did not correct the thrombocytopenia in fear of device thrombosis. She returned for a 2-month follow-up visit with no change in clinical status (persistent gait ataxia). Her international normalized ratio (INR) was maintained between 2 and 2.5. Her platelet count remained between 60 and 70 k/ul. However, a CTA and cerebral angiogram showed increased aneurysm recanalization, compared with the preprocedure images (Fig. 2) 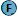 . Cerebral angiogram confirmed that the right vertebral artery ended in the posterior inferior cerebellar artery (PICA). A second PED (3 x 16 mm) was placed uneventfully across the neck of the aneurysm. The aspirin and warfarin regimen was continued on discharge. She did well initially but returned 1 month after her second procedure with aspiration pneumonia. Her INR was 2.2 on admission, and her platelet count was 63 k/ul. CT angiogram and cerebral angiogram (Fig. 3) 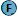 showed massive recanalization with increased flow (now seen as a jet) into the aneurysm . After discussion with the patient and her family, a final retreatment was performed with two additional PEDs (each 3.5 x 20 mm). The final angiographic run showed persistent filling but increased stasis in the aneurysm (Fig. 4) 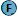 . After treatment, her swallowing function improved along with a 2-week course of high-dose steroids (Decadron, 4 mg oral, every 6 hours). She was discharged back to her rehabilitation facility, tolerating an oral diet and ambulating with assistance. In response to reviewer 4, thrombocytopenia, although rare, is not an unexpected complication in a patient on dual antiplatelet therapy and who also received intravenous heparin during the procedure. It certainly contributed to recanalization In response to reviewer 5, a larger second stent could have been placed to avoid an endoleak. The orifice of the overlapping stents is not tapered. Has this observation been reported previously?This observation has not previously been reported. If your work has Institutional Review Board or other supervisory authority approval, state that now:This work was approved by the Institutional Review Board of the State University of New York at Buffalo. State the source of funding for this study.No financial or material support was received in conjunction with this submission Describe how your observation was made (ie. specific tests, other)This patient was evaluated with serial digital subtraction angiograms showing the regional anatomy and flow into the vertebrobasilar aneurysm. Describe the most important lesson(s) of this report.The PED represents the latest advancement in the treatment of intracranial aneurysms. Although the initial results in the treatment of carotid aneurysms from several large series were excellent, the outcomes of PED in the treatment of vertebrobasilar aneurysms remain largely unknown (6-8). In the Pipeline for the Intracranial Treatment of Aneurysms (PITA) trial, only 2 of the 31 aneurysms treated involved the vertebral artery (1 pre-PICA and 1 at the PICA origin) (7). Szikora et al. (8) reported 1 of 19 aneurysms treated to arise from the basilar trunk. Lylyk et al. (6), in series of 63 aneurysms treated with PED, reported 8 vertebrobasilar aneurysms (4 of which originated at or distal to the PICA). Of the aforementioned 11 vertebrobasilar aneurysms, in only one, a giant fusiform basilar aneurysm previously treated, did PED treatment fail to achieve complete occlusion at 12 months on angiographic follow up. More recently, Lubicz et al. (5), in a series of 27 giant or fusiform aneurysms treated with PED, reported 3 vertebrobasilar aneurysms, with failed complete occlusion in only one case at 6-month angiographic follow up. Similarly, Fischer et al. (3) reported only 1 treatment failure of a basilar trunk aneurysm (among 11 vertebrobasilar aneurysms) secondary to persistent flow through the right vertebral artery. This aneurysm was successfully treated with endovascular sacrifice of the right vertebral artery. In response to reviewer 5, we agree that an endoleak between the original stent and the native vessel is a plausible mechanism for recanalization (a larger device may or may not prevent that). In any case, the PED still served as a flow diverter as it redirected flow away from the aneurysm into the distal basilar artery. For this particular patient, coumadin may ultimately be sufficient as mentioned. This is likely a dissecting aneurysm. Describe the limitations of your findings.The experience in the use of such devices is limited. The main limitation of this report is that it represents the findings of a single case. The use of different size stents may have led to persistence of flow into the aneurysm. Discuss the relevance of your observation to the reader.However, we report here what is to our knowledge the first case of progressive aneurysm recanalization in the vertebrobasilar system despite sequential placement of PEDs across the neck of the aneurysm. Our patient was on therapeutic levels of warfarin with thrombocytopenia during her treatment course, and her anticoagulation status along with thrombocytopenia were undoubtedly in part the cause of recanalization. In the cardiac literature, it is well known that resolution of atrial thrombi typically occurs after weeks of warfarin anticoagulation therapy (1). However, apart from thrombi resolution, persistent inflow into the aneurysm is necessary for recanalization to occur. Each PED offers 30 to 35% surface coverage, and the mechanism of increased or persistent inflow into the aneurysm despite serial PED placement is perplexing. Interestingly, in a canine model of large or giant aneurysms treated with serial telescoping flow diverters, Darsaut et al. (2) showed that aneurysms with perforating branches remained patent despite serial placement of flow diverters, whereas aneurysms without perforators were mainly occluded at angiographic follow up. Of the patent aneurysms, pathologic examination revealed small defects in the neointima formed on the surface of the flow diverter. Th ose authors postulated that in a perforator-rich zone (such as the basilar artery in our case), the demands of the perforators create inflow into the aneurysm through small neointimal defects.Nevertheless, no perforators were demonstrated angiographically on this giant, largely thrombosed aneurysm. Alternatively, in our case, the difference in size between the first (4.25 x 18 mm) and the second (3 x 16 mm) devices could have created an “endoleak” between the two stents (or there could be an endoleak between the first stent and the native vessel), allowing persistent flow into the aneurysm despite the placement of additional PEDs. A larger second stent may or may not have prevented the recanalization. In response to reviewer 1, we did not correct the thrombocytopenia in fear of device thrombosis. We did stop the plavix as our corrective measure. Size mismatch between the first and second stent could have caused blood going between the two stents and entering the aneurysm through the first stent, negating the efficacy of the second stent. In response to reviewer 4, 30-35% coverage is considered quite heavy already (one has to balance treatment efficacy with thromboembolic complications and navigability of using a more metal-heavy stent). No perforators have ever been shown from this giant, largely thrombosed aneurysm. The Pipeline embolization device has shown great initial promise in the treatment of complex intracranial aneurysms. As clinical experience grows, complications associated with Pipeline embolization are increasingly recognized and reported. In this report, we describe what is to our knowledge the first case of progressive recanalization of an aneurysm in the vertebrobasilar system that occurred despite sequential telescoping placement of several Pipeline embolization devices across the neck of the aneurysm. Progressive aneurysm recanalization can occur despite serial telescoping placement of Pipeline embolization devices (PEDs). Anticoagulation status, perforator-rich zone, and size mismatch between stents were the postulated mechanisms for recanalization in our case. Until the technology is fully mature, we must continue to report its complications in a critical and timely fashion. In response to reviewer 5, we have never seen progressive recanalization of any other intracranial aneurysm despite serial PED placement apart from the present case. Flow diverters are the latest tool in the armamentarium in the treatment of intracranial aneurysms.2 Flow diversion is based on the concept of endoluminal reconstruction accomplished by redirecting flow away from the aneurysm through the parent vessel, leading to progressive aneurysm thrombosis and neointimal growth across the aneurysm neck.1 Initial results with the use of these devices were extremely encouraging (6-8)3 ; and in April 2011, the FDA approved the Pipeline embolization device (PED, ev3/Covidien, Irvine, California, USA), a flow diverter, for treatment of large or giant wide-necked intracranial aneurysms from the petrous to superior hypophyseal internal carotid artery (ICA) segments (current on-label indications) (9). Experience with the PED in the treatment of aneurysms in the vertebrobasilar system, however, remains very limited. As clinical experience accumulates, complications associated with PED, both thromboembolic and hemorrhagic, are increasingly recognized (4, 10). We report a case of progressive recanalization of a giant, partially thrombosed basilar artery aneurysm despite serial treatment with PEDs. An 82-year-old woman, who was otherwise healthy, presented initially with gait ataxia. A magnetic resonance (MR) imaging scan and subsequent cerebral angiogram revealed a giant, partially thrombosed midbasilar aneurysm (2.5 x 2.4 cm) with significant brainstem compression (Fig. 1A,B)(Fig. -1), (Fig. -1) . Because of the patient’s age, the amount of mass effect on the brainstem, and the large amount of intra-aneurysmal thrombus demonstrated on MR imaging, Pipeline embolization was thought to be a better treatment option than stent-assisted coil embolization. With stent-assisted coil embolization, we were concerned of thrombus release during coil embolization, a lack of scaffold for the coils due to the large amount of thrombus, and the potential of exacerbating the existing mass effect. After a thorough discussion of therapeutic options and associated risks and benefits with the patient and her family, the decision was made to proceed with PED reconstruction. The patient received loading doses of aspirin and clopidogrel, with therapeutic responses obtained (aspirin response unit of 426 and 50% inhibition on the Plavix assay). A single 4.25 x 18 mm PED was successfully deployed in the basilar artery across the neck of the aneurysm. The patient did well with no new neurologic deficits. However, her hospital course was complicated by thrombocytopenia (platelet count of 60-70 k/ul) (heparin-induced thrombocytopenia panel was negative and the thrombocytopenia was likely antiplatelet-related) and small bilateral pulmonary emboli (asymptomatic) discovered incidentally on follow-up computed tomographic angiogram (CTA) of the aneurysm, which was completely occluded. She was placed on warfarin for pulmonary embolism. Given the patient’s age and her thrombocytopenia, we decided to discontinue the Plavix once she was therapeutic on warfarin. She was subsequently discharged to a rehabilitation facility on aspirin and warfarin. We did not correct the thrombocytopenia in fear of device thrombosis. She returned for a 2-month follow-up visit with no change in clinical status (persistent gait ataxia). Her international normalized ratio (INR) was maintained between 2 and 2.5. Her platelet count remained between 60 and 70 k/ul. However, a CTA and cerebral angiogram showed increased aneurysm recanalization, compared with the preprocedure images (Fig. 2)(Fig. -1) . Cerebral angiogram confirmed that the right vertebral artery ended in the posterior inferior cerebellar artery (PICA). A second PED (3 x 16 mm) was placed uneventfully across the neck of the aneurysm. The aspirin and warfarin regimen was continued on discharge. She did well initially but returned 1 month after her second procedure with aspiration pneumonia. Her INR was 2.2 on admission, and her platelet count was 63 k/ul. CT angiogram and cerebral angiogram (Fig. 3)(Fig. -1) showed massive recanalization with increased flow (now seen as a jet) into the aneurysm. After discussion with the patient and her family, a final retreatment was performed with two additional PEDs (each 3.5 x 20 mm). The final angiographic run showed persistent filling but increased stasis in the aneurysm (Fig. 4)(Fig. -1) . After treatment, her swallowing function improved along with a 2-week course of high-dose steroids (Decadron, 4 mg oral, every 6 hours). She was discharged back to her rehabilitation facility, tolerating an oral diet and ambulating with assistance.
In response to reviewer 4, thrombocytopenia, although rare, is not an unexpected complication in a patient on dual antiplatelet therapy and who also received intravenous heparin during the procedure. It certainly contributed to recanalization In response to reviewer 5, a larger second stent could have been placed to avoid an endoleak. The orifice of the overlapping stents is not tapered. This observation has not previously been reported. Figure 1: 1a
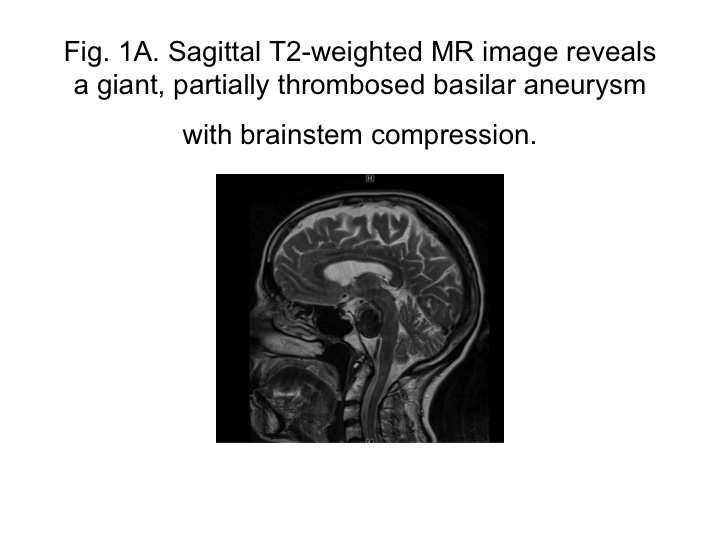 |
| Sagittal T2-weighted MR image reveals a giant, partially thrombosed basilar aneurysm with brainstem compression. |
Figure 2: 1b
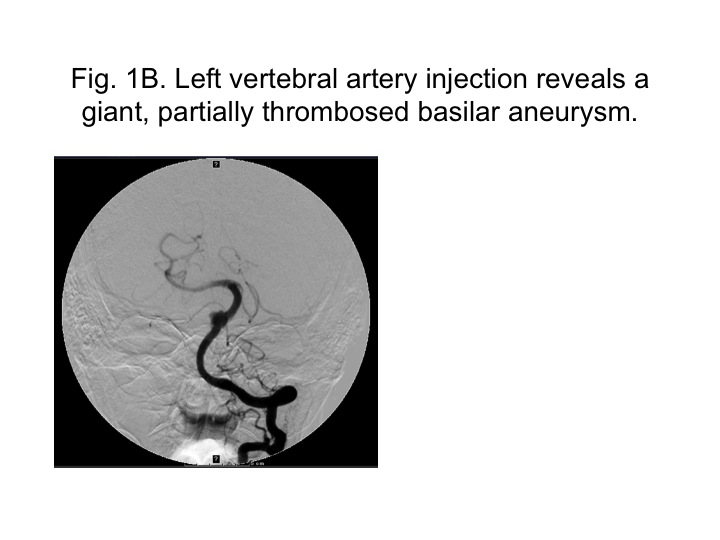 |
| Left vertebral artery injection reveals a giant, partially thrombosed basilar aneurysm. |
Figure 3: 2
Figure 4: 3
Figure 5: 4
This work was approved by the Institutional Review Board of the State University of New York at Buffalo.
No financial or material support was received in conjunction with this submission This patient was evaluated with serial digital subtraction angiograms showing the regional anatomy and flow into the vertebrobasilar aneurysm. The PED represents the latest advancement in the treatment of intracranial aneurysms. Although the initial results in the treatment of carotid aneurysms from several large series were excellent, the outcomes of PED in the treatment of vertebrobasilar aneurysms remain largely unknown (6-8). In the Pipeline for the Intracranial Treatment of Aneurysms (PITA) trial, only 2 of the 31 aneurysms treated involved the vertebral artery (1 pre-PICA and 1 at the PICA origin) (7). Szikora et al. (8) reported 1 of 19 aneurysms treated to arise from the basilar trunk. Lylyk et al. (6), in series of 63 aneurysms treated with PED, reported 8 vertebrobasilar aneurysms (4 of which originated at or distal to the PICA). Of the aforementioned 11 vertebrobasilar aneurysms, in only one, a giant fusiform basilar aneurysm previously treated, did PED treatment fail to achieve complete occlusion at 12 months on angiographic follow up. More recently, Lubicz et al. (5), in a series of 27 giant or fusiform aneurysms treated with PED, reported 3 vertebrobasilar aneurysms, with failed complete occlusion in only one case at 6-month angiographic follow up. Similarly, Fischer et al. (3) reported only 1 treatment failure of a basilar trunk aneurysm (among 11 vertebrobasilar aneurysms) secondary to persistent flow through the right vertebral artery. This aneurysm was successfully treated with endovascular sacrifice of the right vertebral artery. In response to reviewer 5, we agree that an endoleak between the original stent and the native vessel is a plausible mechanism for recanalization (a larger device may or may not prevent that). In any case, the PED still served as a flow diverter as it redirected flow away from the aneurysm into the distal basilar artery. For this particular patient, coumadin may ultimately be sufficient as mentioned. This is likely a dissecting aneurysm.
The experience in the use of such devices is limited. The main limitation of this report is that it represents the findings of a single case. The use of different size stents may have led to persistence of flow into the aneurysm.
However, we report here what is to our knowledge the first case of progressive aneurysm recanalization in the vertebrobasilar system despite sequential placement of PEDs across the neck of the aneurysm. Our patient was on therapeutic levels of warfarin with thrombocytopenia during her treatment course, and her anticoagulation status along with thrombocytopenia were undoubtedly in part the cause of recanalization. In the cardiac literature, it is well known that resolution of atrial thrombi typically occurs after weeks of warfarin anticoagulation therapy (1). However, apart from thrombi resolution, persistent inflow into the aneurysm is necessary for recanalization to occur. Each PED offers 30 to 35% surface coverage, and the mechanism of increased or persistent inflow into the aneurysm despite serial PED placement is perplexing. Interestingly, in a canine model of large or giant aneurysms treated with serial telescoping flow diverters, Darsaut et al. (2) showed that aneurysms with perforating branches remained patent despite serial placement of flow diverters, whereas aneurysms without perforators were mainly occluded at angiographic follow up. Of the patent aneurysms, pathologic examination revealed small defects in the neointima formed on the surface of the flow diverter. Those authors postulated that in a perforator-rich zone (such as the basilar artery in our case), the demands of the perforators create inflow into the aneurysm through small neointimal defects.Nevertheless, no perforators were demonstrated angiographically on this giant, largely thrombosed aneurysm. Alternatively, in our case, the difference in size between the first (4.25 x 18 mm) and the second (3 x 16 mm) devices could have created an “endoleak” between the two stents (or there could be an endoleak between the first stent and the native vessel), allowing persistent flow into the aneurysm despite the placement of additional PEDs. A larger second stent may or may not have prevented the recanalization. In response to reviewer 1, we did not correct the thrombocytopenia in fear of device thrombosis. We did stop the plavix as our corrective measure. Size mismatch between the first and second stent could have caused blood going between the two stents and entering the aneurysm through the first stent, negating the efficacy of the second stent. In response to reviewer 4, 30-35% coverage is considered quite heavy already (one has to balance treatment efficacy with thromboembolic complications and navigability of using a more metal-heavy stent). No perforators have ever been shown from this giant, largely thrombosed aneurysm. The Author(s) wish to thank: Paul H. Dressel BFA for assistance with preparation of the illustration and Debra J. Zimmer, AAS CMA-A for editorial assistance.
Nothing to disclose: Dr. Dumont and Dr Kan. Dr. Levy receives research grant support (principal investigator: Stent-Assisted Recanalization in acute Ischemic Stroke, SARIS), other research support (devices), and honoraria from Boston Scientific* and research support from Codman & Shurtleff, Inc. and ev3/Covidien Vascular Therapies; has ownership interests in Intratech Medical Ltd. and Mynx/Access Closure; serves as a consultant on the board of Scientific Advisors to Codman & Shurtleff, Inc.; serves as a consultant per project and/or per hour for Codman & Shurtleff, Inc., ev3/Covidien Vascular Therapies, and TheraSyn Sensors, Inc.; and receives fees for carotid stent training from Abbott Vascular and ev3/Covidien Vascular Therapies. Dr. Levy receives no consulting salary arrangements. All consulting is per project and/or per hour.Project Roles:
1. Darsaut T, E.,Bing, F.,Salazkin, I.,Gevry, G.,Raymond, J., Testing Flow Diverters in Giant Fusiform Aneurysms: A New Experimental Model Can Show Leaks Responsible for Failures.. Ajnr American J Neuroradiology : - , 20112. Fischer, Sebastian.,Vajda, Zsolt.,Aguilar perez, Marta.,Schmid, Elisabeth.,Hopf, Nikolai.,B?zner, Hansj?rg., et al: Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections.. Neuroradiology : - , 20113. Lubicz, Boris.,Collignon, Laurent.,Raphaeli, Ga?.,De witte, Olivier., Pipeline flow-diverter stent for endovascular treatment of intracranial aneurysms: preliminary experience in 20 patients with 27 aneurysms.. World Neurosurg 76(1-2): 114 - 119, 20114. Lylyk, Pedro.,Miranda, Carlos.,Ceratto, Rosana.,Ferrario, Angel.,Scrivano, Esteban.,Luna Hugh, Ramirez., et al: Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience.. Neurosurgery 64(4): 632 - 42; discussion 642, 20095. Nelson P, K.,Lylyk, P.,Szikora, I.,Wetzel S, G.,Wanke, I.,Fiorella, D., The pipeline embolization device for the intracranial treatment of aneurysms trial.. AJNR Am J Neuroradiol 32(1): 34 - 40, 2011
|

