Long Term (>20 Years) Outcome Assessment of Vestibular Schwannoma Patients treated by Gamma Knife RadiosurgeryKeywords: gamma knife, vestibular schwannoma, radiosurgery, outcome, stereotactic radiosurgeryInteractive ManuscriptAsk Questions of this Manuscript: What is the background behind your study?Gamma knife radiosurgery is commonly used for the care of patients with vestibular schwannomas. However, long-term results regarding prevention of tumor growth, avoidance of new neurological deficits, and associated morbidity is limited. What is the purpose of your study?Our purpose was to evaluate long term outcomes of patients who underwent stereotactic gamma knife radiosurgery for vestibular schwannoma by assessing clinical and imaging outcomes. Describe what you did.We reviewed the records of 78 consecutive patients who underwent gamma knife stereotactic radiosurgery at the University of Pittsburgh Medical Center for a vestibular schwannoma between 1987 and 1989. Describe your patient group.The patient population consisted of 33 (42%) men and 45 (58%) women at a median age of 59 years (range, 15 to 83 years). Three (4%) patients had a prior subtotal resection, 13 (17%) had a prior total resection, and no patient had a prior history of radiation therapy. Twelve patients were diagnosed with neurofibromatosis type II but had only one tumor irradiated. The median marginal dose and percent isodose was 18 Gy and 50%, respectively. The median target volume was 3.13 cm 2 and varied from 0.1 to 14.3 cm 2. Describe your main findings.The median survival was 249 months. Forty-four of 78 patients (56%) died from causes unrelated to their vestibular schwannoma. Clinical follow-up varied from 4 to 292 months (median, 66 months), and 12 (15%) patients had a clinical assessment past 20 years. Imaging follow-up varied from 4 to 276 months (median, 91 months) and 5 (6%) patients had an imaging evaluation past 20 years. Of the 78 tumors, 54 regressed in size, 19 remained stable, and 5 enlarged. The 20-year actuarial tumor freedom from progression and freedom from resection rate was 92% ± 3.5% (rate ± standard error). All 5 of the progressing tumors were resected after gamma knife radiosurgery and were the only resected tumors in this series. Using an 18 Gy median dose in 44 patients with hearing, preservation rates were 30% for maintainance of the same Gardner Robertson level, 44% for maintaining serviceable hearing, and 48% for maintaining any level of hearing. The 20-year actuarial rate of freedom from new temporary or permanent facial neuropathy, excluding patients with House-Brackmann grade 6 facial neuropathy prior to radiosurgery, was 59% ± 6%. The 20-year actuarial rate of freedom from new temporary or permanent trigeminal neuropathy was 54.6% ± 6%. Describe the main limitation of this study.The main limitation of this study is the low rate of imaging evaluations past 15 years, mainly due to the advanced age of patients managed with radiosurgery in our early experience. In addition, these results may not be applicable to later patients managed with a dose of 12-14Gy. Describe your main conclusion.Gamma knife radiosurgery can provide long term tumor control with low risk for early or delayed morbidity in the care of patients with vestibular schwannoma. Describe the importance of your findings and how they can be used by others. This data is important because late (10-23 years) outcomes were similar to early (5-10 years) outcomes. What is the importance to the reader/community?Patient perspectives as they continue life beyond treatment remains an important consideration for vestibular schwannomas, where the most common morbidities include hearing impairment, facial and trigeminal neuropathy, and balance problems. Long-term analysis is beneficial in providing patients and physicians information regarding these morbidities. What is your hypothesis? (What question(s) did you ask?)Therefore, it is our goal to describe our experience with gamma knife radiosurgery of vestibular schwannomas. This study evaluates the long-term durability of gamma knife radiosurgery in the care of patients with vestibular schwannomas, particularly in regard to cessation of tumor growth, avoidance of new neurological deficits, and associated morbidity. Our hypothesis is that tumor control and morbidity will be similar between evaluable patients with 5-10 and 10-20 year follow-up. What was done in your study?We reviewed clinical evaluations and serial imaging studies for 78 consecutive patients treated at the University of Pittsburgh Medical Center between 1987 and 1989. What are the main limitations of your research method?The main limitation of this study is the low rate of imaging evaluations past 15 years, mainly due to the advanced age of patients managed with radiosurgery in our early experience. In addition, this series reflects outcomes using techniques no longer practiced with higher radiosurgery doses, non image-integrated dose planning, and targeting that was typically facilitated by CT imaging only. We do not think that current imaging allows better detection of treatment failure. Although the radiobiology of solitary versus neurofibromatosis type 2 tumors may be different, we do not have evidence to support that conclusion. Similarly, a partially resected tumor and a primarily radiated tumor may have a different biology (due to possible changes in vascularity after resection), but this is also unclear. If your work has Institution Review Board or any other supervisory authority approval, state that now:The University of Pittsburgh Institutional Review Board approved this study. State the source of funding for this study.This work was not funded by any external source. Describe patient age (mean, range)
Gamma knife stereotactic radiosurgery was performed at the University of Pittsburgh Medical Center between 1987 and 1989 for 78 consecutive patients diagnosed with vestibular schwannomas. The median patient age was 58.5 years (range, 15 to 83 years) and the ages at the time of radiosurgery are described in figure 1.   Describe patient sex (number male and number female)Forty-five (58%) patients were female, and 33 (42%) were male. Describe other important patient features (symptoms (list); clinical presentation features, prior treatment, employment, etc)All patients had imaging features that were typical of vestibular schwannomas, or had prior histological confirmation of a vestibular schwannoma. Describe other important patient features (symptoms (list); clinical presentation features, prior treatment, employment, etc)All patients had imaging features that were typical of vestibular schwannomas, or had prior histological confirmation of a vestibular schwannoma. Describe disease features (example = tumor subtypes)Twelve patients were previously diagnosed with neurofibromatosis type 2. Three patients had 3 gross total surgical resections of their vestibular schwannomas prior to radiosurgery. These tumors had imaging-defined recurrences. Thirteen patients had 26 subtotal surgical resections: 6 patients had 1 resection, 3 patients had 2 resections, 2 patients had 3 resections, and 2 patients had 4 resections. No patient had a history of fractionated radiation therapy or SRS for their vestibular schwannoma. Describe disease features (example = tumor subtypes)Twelve patients were previously diagnosed with neurofibromatosis type 2. Three patients had 3 gross total surgical resections of their vestibular schwannomas prior to radiosurgery. These tumors had imaging-defined recurrences. Thirteen patients had 26 subtotal surgical resections: 6 patients had 1 resection, 3 patients had 2 resections, 2 patients had 3 resections, and 2 patients had 4 resections. No patient had a history of fractionated radiation therapy or SRS for their vestibular schwannoma. Describe the clinical intervention (ie. medications, devices, techniques)
Stereotactic radiosurgery was performed using the Leksell Gamma Knife (Elekta Instruments, Norcross, GA). Tumors were defined by computed tomography imaging with a stereotactic frame in place on the day of the procedure. Radiosurgery was performed under local anesthesia and was supplemented with intravenous sedation when necessary. Multiple isocenters were used when necessary to create an optimized individual dose plan. The median marginal dose was 18 Gy (range 14 to 32 Gy), a dose much higher than used currently. Target tumor volume varied from .1 cm 3 to 14.3 cm 3 (median, 3.13 cm 3). Dose planning was performed with CT imaging. Patients were given a single 20-40 mg dose of intravenous methylprednisone after radiosurgery and were discharged from the hospital the following morning.
Serial imaging studies (MRI, or CT when MRI was contraindicated or unavailable) were used to define changes in tumor volume and were requested every 6 months for the first two years, annually for the next two years, and then once every two years for the remainder of the follow-up period. Five caliper measurements of each tumor were taken according to previously described methods  and the measurements were compared to previous and preoperative imaging to determine changes in size. Growth or regression were defined as a 2 mm change in size in any one dimension or a 1 mm change in size in any two dimensions of the tumor. Tumors that showed transient expansion and later regression were coded as regressed. Hydrocephalus or any changes around the tumor, if present, were identified at every imaging evaluation as well. Clinical and imaging evaluations were performed at our center for the patients living in the Pittsburgh region and by the referring physicians for patients who lived far away. Each clinical evaluation involved detailed testing that included testing of cranial nerve function and evaluation of the peripheral and central nervous system.
Describe the tests used to perform your research (Imaging, Patient Outcomes, Other specific tests.)
Hearing preservation, facial neuropathy, and trigeminal neuropathy were also examined for this study. Hearing preservation was assessed in patients with testable hearing prior to radiosurgery and was determined by audiologic evaluation performed in 6 to 12 month intervals. The Gardner-Robertson hearing scale was used to classify hearing based on speech discrimination scores (SDS) and pure tone average (PTA). Serviceable hearing was defined as Gardner-Robertson grade 1 or grade 2 hearing (SDS > 50% and PTA <50 dB). Hearing was assessed prior to radiosurgery as follows: 9 patients had grade 1 hearing, 9 patients had grade 2 hearing, 22 patients had grade 3 hearing, 4 patients had grade 4 hearing, and 34 patients had grade 5 hearing. Patients with no testable hearing (grade 5) were excluded from hearing preservation analyses. Follow-up endpoints for hearing preservation were maintenance of same class hearing, serviceable hearing, or any testable hearing.
Facial neuropathy was defined as any temporary or permanent decline in facial nerve function, as measured by the House-Brackmann grading scale. Prior to radiosurgery, facial nerve function was normal (House-Brackmann grade 1) in 57 patients, grade 2 in 8 patients, grade 3 in 5 patients, grade 4 in 3 patients, grade 5 in 2 patients, and grade 6 (no facial nerve function) in 3 patients. Trigeminal neuropathy was defined as any temporary or permanent decline in trigeminal nerve function, subjectively noticed by the patient or objectively measured by the examining physician. We did not record data on corneal anesthesia. What percent of study subjects completed each of the tests?
Clinical follow-up for this series varied from 4 to 292 months (median, 66 months). Twelve (15%) patients had a clinical follow-up greater than 20 years. Imaging follow-up ranged from 4 to 276 months (median, 91 months), and 5 (6%) patients had an imaging follow-up greater than 20 years. These five patients also had a clinical follow-up greater than 20 years. Sixty-eight and 73 patients were lost to clinical and imaging follow-up, respectively. Sixteen of these patients were lost because they died within their follow-up period (within 3 years of their latest clinical or imaging follow-up date). The remainder of the patients were lost to follow-up through loss of contact, moving, and financial and insurance issues. Multiple attempts to contact patients lost to follow-up were made by phone to inquire about their clinical and imaging status.
Describe who conducted the tests. (Study investigators or other parties?)All data was evaluated by neurosurgeons and radiation oncologists and then entered into a prospective registry as it was obtained. Were the tests validated for use in this kind of study?All study tests were validated for their utility. Describe your statistical methods or tests usedFor statistical analysis, we constructed Kaplan-Meier plots for tumor freedom from progression and freedom from neurological deficit using the dates of first SRS, follow-up MRIs, and death or last follow-up. Standard statistical processing software (SPSS, version 17.0, SPSS Inc., Chicago, IL) was used. Describe your study power calculation (if any)This study did not include a power calculation. Describe your chosen level of statistical significanceWe did not use a level of statistical significance. Provide the results for the most important outcome of your research [i.e. Patient survival]The median survival for the entire group of patients was 249 months (20.8 years). 
Among the 44 (56%) patients who died, median survival was 156 months.
Tumor response to radiosurgery was favorable in most of the
patients, with tumors remaining stable, regressing, and enlarging in 19 (24%),
54 (69%), and 5 (6%) of patients, respectively (Figure 2). Of the 5 enlarging
tumors, one was in a patient diagnosed with NF2, one was later diagnosed as a malignant neurofibrosarcoma after SRS,
and another developed a post-radiosurgery cyst after having also undergone a subtotal resection prior to radiosurgery. All five of the patients with
enlarging tumors underwent resection of their tumor at a mean of 35 months after radiosurgery. Image defined growth was seen 2, 5, 34, 35, and 72 months after radiosurgery. The patient with tumor growth within 2 months after radiosurgery developed brainstem compression and House-Brackmann grade 4 facial nerve function 1 week after resection of the tumor. The tumor that developed a cyst at 34 months was stereotactically drained followed by surgical resection, and the patient underwent a second gamma knife radiosurgery procedure for the remnant. The tumor that progressed 72 months after radiosurgery was diagnosed as a triton tumor after resection and has been published previously. The 20-year
actuarial rate for tumor freedom from progression was 92% ± 3.5% (rate ± standard error) (Figure 3).
Four patients developed new tumors following radiosurgery at locations unrelated to the initial radiosurgical site. Two pituitary adenomas developed in two patients 119 months and 124 months after radiosurgery. Both patients had a history of recurrent pituitary adenoma despite resection, and both adenomas were surgically resected. Two patients developed meningiomas 74 months and 143 months after radiosurgery. The first meningioma developed on the left petrous ridge in an NF2 patient treated for a right vestibular schwannoma. The second meningioma developed at the right parietal parasagittal area in a patient managed for a right vestibular schwannoma. These patients had previously been surveyed regarding outcomes and satisfaction during a 5-10 year review but we did not repeat that survey in this longer analysis.    Discuss any additional outcomes of your study [i.e. Imaging findings, Patient functional outcomes, Complications]Patients who did not have any hearing prior to SRS (n = 34) were
not included in this hearing analysis. In the remaining 44 patients,
Gardner-Robertson hearing class was preserved in 13 (30%) patients and any
hearing, defined as Gardner-Robertson classes 1-4, was preserved in 21 (48%) of
patients.   The 20-year actuarial rates of preservation of any hearing in patients with tumor volumes <1 cm 3 was 54% ± 15.4% (n=12); between 1 and 3 cm 3 was 62.3% ± 15% (n=11); and >3 cm 3 was 29.1% ± 10.6% (n=21). The 20-year actuarial rates of preservation of same class hearing in patients with tumor volumes <1 cm 3 was 33.3% ± 15.1% (n=12); between 1 and 3 cm 3 was 53% ± 15.5% (n=11); and >3 cm 3 was 16.3% ± 8.4% (n=21).
Serviceable hearing, defined as Gardner-Robertson classes 1 and
2, was preserved in 8 (44%) out of 18 patients. The 20-year actuarial rates of preservation of serviceable hearing in patients with tumor volumes <1 cm 3 was 100% (n=3); between 1 and 3 cm 3 was 66.7% ± 19.2% (n=6); and >3 cm 3 was 11.1% ± 10.5% (n=9). All of the 3 patients with tumor volumes <1 cm 3 maintained serviceable hearing.
20-year actuarial hearing preservation rates over all tumor volumes for same class hearing, serviceable hearing, and any testable hearing were 30% ± 7.3%, 43% ± 12%, and 44% ± 7.9%, respectively (Figures 4, 5, and 6).
Hearing class improved in 3 patients between 3 and 28 months after
treatment. The improvement in one patient from class 4 to class 3 was
temporary, lasting only 6 months after which the patient became class 5. 
Facial neuropathy was defined as any temporary or permanent
decrease in facial nerve function as defined by an increase in House-Brackmann
grade. Three patients had HB grade 6 facial function prior to radiosurgery.
 Twenty-nine patients developed facial nerve neuropathy between 3 days and 72
months after SRS. Normal facial nerve function was preserved in 40
of 75 patients who could be evaluated and in 39 of 59 patients with normal
facial nerve function before radiosurgery. The 20-year actuarial rate of freedom from new facial neuropathy, excluding patients with House-Brackmann grade 6 facial neuropathy prior to radiosurgery, was 59% ± 6% (Figure 7). In patients with tumor volumes <1 cm 3 the rate was 76.6% ± 11.9% (n=14); between 1 and 3 cm 3 was 74.4% ± 9% (n=24); and >3 cm 3 was 45.6% ± 8.7% (n=40).
Trigeminal neuropathy was defined as any temporary or permanent
decrease in facial sensation, measured objectively by the physician or
subjectively by the patient during patient interview or examination.  Twenty-four patients had a trigeminal nerve deficit prior to radiosurgery. Thirty-three patients developed a new or worsened trigeminal nerve deficit between 2 and 63
months of radiosurgery for a 20-year actuarial rate of 54.6%
± 6% for freedom from new trigeminal nerve deficit (Figure 8). In patients with tumor volumes <1 cm 3 the rate was 90% ± 9.5% (n=14); between 1 and 3 cm 3 was 57.2% ± 10.3% (n=24); and >3 cm 3 was 42.4% ± 8.2% (n=40). Two patients experienced trigeminal neuralgia during their follow-up. The first patient had pain prior to radiosurgery that is still
intermittently present. The second patient developed trigeminal neuralgia 3 months after radiosurgery, which subsequently resolved 32 months later.
Radiation related injury may have developed in one NF2 patient with bilateral schwannomas and a fifth nerve tumor ipsilateral to the radiosurgically treated vestibular schwannoma. Imaging performed 3 months after radiosurgery showed typical central tumor necrosis and the patient subsequently developed a trigeminal deficit 5 months after radiosurgery. The patient was lost to follow-up shortly after, and so it is uncertain if the deficit was temporary or permanent. Discuss Statistical Outcomes [i.e. Multivariate analyses]No comparative statistics were performed. Discuss Statistical Outcomes [i.e. Multivariate analyses]No comparative statistics were performed. Provide the background and reason for your work and briefly summarize important prior research.Over the last 25 years, stereotactic radiosurgery has become one of the most important therapeutic options for vestibular schwannoma patients. Nevertheless, the majority of reports provide outcomes over the first few years following the procedure. Such data can be particularly important in an analysis of morbidity but not in terms of long-term tumor response. This study found similar outcomes between late (10-23 years) and earlier (5-10 years) reports for the same subset of subjects. Data early in our experience showed that 55% of tumors regressed and 42% of tumors remained stable in patients with at least 1 year of follow-up, for a total imaging-defined tumor control rate of 97%.  Foote et. al. reported an imaging-defined tumor control rate of 100% for small and stable tumors.  Norén found a tumor control rate of 96.7%.  Another study, where marginal tumor doses were lowered to an average of 13 Gy, showed a similar tumor control rate defined by freedom from resection of 97% at 5 years post-radiosurgery. 7 Our experience at 10 years was also similar to our early experience as the resection free tumor control rate was 98% and the imaging-defined tumor control rate was 94%.  Hearing preservation has improved as margin doses have been reduced from 18-20 Gy prior to 1992 to the more current 12-13 Gy. Early studies of patients irradiated using a median margin dose of 16-20 Gy or 14-20 Gy reported a 2-year actuarial serviceable hearing preservation rate of 41% and 46%, respectively.   More recent studies, where median margin doses were 13 Gy, have reported serviceable hearing rates of 73%. 7 In comparison, hearing preservation after resection are typically lower but vary from 30% to 80%.  Trigeminal and facial neuropathies typically occur within the first few years of radiosurgery. Early in our experience we reported rates of new facial and trigeminal neuropathies as 30% and 29%, respectively.  Lower rates have been documented at a less then 5% risk of new facial or trigeminal neuropathy when margin doses have been decreased to 13 Gy.   ,7, ,7,
It is important to note that the approach and results documented in this report do not represent current methods. Indeed, the use of CT-based targeting was replaced with MRI in the early 1990s, and improved outcomes followed. Much has evolved in technology, imaging, and dose planning over the past 20 years. Discuss the most important findings in your study.The data from this report suggests that long-term tumor control from 10 to 23 years was similar to early control. Tumor freedom from progression stabilizes 5 years after radiosurgery, suggesting that tumors that are destined to fail will resume their natural tendency to grow within a few years of being irradiated. Once this 5 year point has passed, it is unlikely a dormant tumor will begin to grow again.
Cranial nerve preservation rates were lower than in more recent reports after 1995 because this study involved patients managed early in our experience when the patients were older, the tumor margin dose averaged 18 Gy compared to the cranial nerve sparing doses of 12-13 Gy used today, and target definition was mainly with CT rather than MRI. However, our data still shows that similar to tumor size, changes in hearing occur within the first few years of radiosurgery and stabilize thereafter. Rates of preservation of same class, serviceable, and testable hearing beyond 10 years are similar to rates between 5 and 10 years. Facial and trigeminal nerve preservation followed a similar pattern. Discuss the various aspects of your work (for example, treatment-related complications, comparisons to other approaches or techniques, cost-effectiveness analysis)Much has changed in radiosurgery technique since these patients were cared for before 1992. Beginning around 1994, numerous aspects evolved. As noted above, targeting and dose planning included MR imaging. Margin doses were reduced to the current range of 12-13 Gy. Radiosurgical dose planning with the gamma knife utilized images integrated into the dose planning software (GammaPlan, Elekta). It became more common to offer radiosurgery to younger patients, and subsequent studies have shown that younger patients have higher hearing preservation rates.   Studies in the last decade indicated the importance of cochlear dose for hearing preservation.  Recently, the Perfexion gamma knife allowed more efficient use of beam blocking if desired for individual patients. Discuss Future Work and Recommendations.We can conclude that the long-term data suggests that tumor progression and neurological deficits stabilize past five years in the long-term for patients with margin doses between 18 and 20 Gy. We remain vigilant with continuing follow-up assessments so that we can report the outcomes past twenty years in patients managed with current tumor margin doses, high resolution imaging-based planning, and careful attention to regional structures such as the cochlea. The results of this study do not represent what can be achieved with current techniques, but serve as a historical foundation for work in this field. Gamma knife radiosurgery is commonly used for the care of patients with vestibular schwannomas. However, long-term results regarding prevention of tumor growth, avoidance of new neurological deficits, and associated morbidity is limited. Our purpose was to evaluate long term outcomes of patients who underwent stereotactic gamma knife radiosurgery for vestibular schwannoma by assessing clinical and imaging outcomes. We reviewed the records of 78 consecutive patients who underwent gamma knife stereotactic radiosurgery at the University of Pittsburgh Medical Center for a vestibular schwannoma between 1987 and 1989. The patient population consisted of 33 (42%) men and 45 (58%) women at a median age of 59 years (range, 15 to 83 years). Three (4%) patients had a prior subtotal resection, 13 (17%) had a prior total resection, and no patient had a prior history of radiation therapy. Twelve patients were diagnosed with neurofibromatosis type II but had only one tumor irradiated. The median marginal dose and percent isodose was 18 Gy and 50%, respectively. The median target volume was 3.13 cm2 and varied from 0.1 to 14.3 cm2. The median survival was 249 months. Forty-four of 78 patients (56%) died from causes unrelated to their vestibular schwannoma. Clinical follow-up varied from 4 to 292 months (median, 66 months), and 12 (15%) patients had a clinical assessment past 20 years. Imaging follow-up varied from 4 to 276 months (median, 91 months) and 5 (6%) patients had an imaging evaluation past 20 years. Of the 78 tumors, 54 regressed in size, 19 remained stable, and 5 enlarged. The 20-year actuarial tumor freedom from progression and freedom from resection rate was 92% ± 3.5% (rate ± standard error). All 5 of the progressing tumors were resected after gamma knife radiosurgery and were the only resected tumors in this series. Using an 18 Gy median dose in 44 patients with hearing, preservation rates were 30% for maintainance of the same Gardner Robertson level, 44% for maintaining serviceable hearing, and 48% for maintaining any level of hearing. The 20-year actuarial rate of freedom from new temporary or permanent facial neuropathy, excluding patients with House-Brackmann grade 6 facial neuropathy prior to radiosurgery, was 59% ± 6%. The 20-year actuarial rate of freedom from new temporary or permanent trigeminal neuropathy was 54.6% ± 6%. The main limitation of this study is the low rate of imaging evaluations past 15 years, mainly due to the advanced age of patients managed with radiosurgery in our early experience. In addition, these results may not be applicable to later patients managed with a dose of 12-14Gy. Gamma knife radiosurgery can provide long term tumor control with low risk for early or delayed morbidity in the care of patients with vestibular schwannoma. This data is important because late (10-23 years) outcomes were similar to early (5-10 years) outcomes. Radiosurgical treatment of vestibular schwannomas has recently become a popular alternative to microsurgical resection. However, data from serial imaging studies and clinical follow ups to assess long-term outcomes are rare. Most of the early literature that reported tumor control and freedom of new neurological deficits focused on short term results immediately after radiosurgery1,3,4,7,8,13,14,16,17,21,22,24,26,27,29,30 and long-term microsurgical data for comparison is rare as well.9,25,31,32,33 Recently, data regarding 10 year outcomes is emerging as investigators further publish their experiences.2,3,10,15,18,23 However, long-term results beyond 10 years remain to be seen. Patient perspectives as they continue life beyond treatment remains an important consideration for vestibular schwannomas, where the most common morbidities include hearing impairment, facial and trigeminal neuropathy, and balance problems. Long-term analysis is beneficial in providing patients and physicians information regarding these morbidities. Therefore, it is our goal to describe our experience with gamma knife radiosurgery of vestibular schwannomas. This study evaluates the long-term durability of gamma knife radiosurgery in the care of patients with vestibular schwannomas, particularly in regard to cessation of tumor growth, avoidance of new neurological deficits, and associated morbidity. Our hypothesis is that tumor control and morbidity will be similar between evaluable patients with 5-10 and 10-20 year follow-up. We reviewed clinical evaluations and serial imaging studies for 78 consecutive patients treated at the University of Pittsburgh Medical Center between 1987 and 1989. Short term reports of radiosurgery for vestibular schwannomas show promising tumor control, avoidance of new neurological deficits, and few associated morbidities.7,12,24 Tumor control at 5 years or less after radiosurgery is nearly 100%;7,8 at 10 years it has been reported as above 87%.2,3,10,15,18,24 Reports discussing cranial nerve preservation as far out as 10 years after radiosurgery describe facial nerve and trigeminal nerve preservation as 99% and 97%, respectively, for tumors with margin doses of 12-13 Gy.3 Rates of hearing preservation, facial neuropathy, and trigeminal neuropathy have all been found to be related to margin dose.3,20 The main limitation of this study is the low rate of imaging evaluations past 15 years, mainly due to the advanced age of patients managed with radiosurgery in our early experience. In addition, this series reflects outcomes using techniques no longer practiced with higher radiosurgery doses, non image-integrated dose planning, and targeting that was typically facilitated by CT imaging only. We do not think that current imaging allows better detection of treatment failure. Although the radiobiology of solitary versus neurofibromatosis type 2 tumors may be different, we do not have evidence to support that conclusion. Similarly, a partially resected tumor and a primarily radiated tumor may have a different biology (due to possible changes in vascularity after resection), but this is also unclear. The University of Pittsburgh Institutional Review Board approved this study. This work was not funded by any external source.
Gamma knife stereotactic radiosurgery was performed at the University of Pittsburgh Medical Center between 1987 and 1989 for 78 consecutive patients diagnosed with vestibular schwannomas. The median patient age was 58.5 years (range, 15 to 83 years) and the ages at the time of radiosurgery are described in figure 1.(DS. -1), (Fig. -1)
Forty-five (58%) patients were female, and 33 (42%) were male. All patients had imaging features that were typical of vestibular schwannomas, or had prior histological confirmation of a vestibular schwannoma. All patients had imaging features that were typical of vestibular schwannomas, or had prior histological confirmation of a vestibular schwannoma. Twelve patients were previously diagnosed with neurofibromatosis type 2. Three patients had 3 gross total surgical resections of their vestibular schwannomas prior to radiosurgery. These tumors had imaging-defined recurrences. Thirteen patients had 26 subtotal surgical resections: 6 patients had 1 resection, 3 patients had 2 resections, 2 patients had 3 resections, and 2 patients had 4 resections. No patient had a history of fractionated radiation therapy or SRS for their vestibular schwannoma. Twelve patients were previously diagnosed with neurofibromatosis type 2. Three patients had 3 gross total surgical resections of their vestibular schwannomas prior to radiosurgery. These tumors had imaging-defined recurrences. Thirteen patients had 26 subtotal surgical resections: 6 patients had 1 resection, 3 patients had 2 resections, 2 patients had 3 resections, and 2 patients had 4 resections. No patient had a history of fractionated radiation therapy or SRS for their vestibular schwannoma.
Stereotactic radiosurgery was performed using the Leksell Gamma Knife (Elekta Instruments, Norcross, GA). Tumors were defined by computed tomography imaging with a stereotactic frame in place on the day of the procedure. Radiosurgery was performed under local anesthesia and was supplemented with intravenous sedation when necessary. Multiple isocenters were used when necessary to create an optimized individual dose plan. The median marginal dose was 18 Gy (range 14 to 32 Gy), a dose much higher than used currently. Target tumor volume varied from .1 cm3 to 14.3 cm3 (median, 3.13 cm3). Dose planning was performed with CT imaging. Patients were given a single 20-40 mg dose of intravenous methylprednisone after radiosurgery and were discharged from the hospital the following morning.
Serial imaging studies (MRI, or CT when MRI was contraindicated or unavailable) were used to define changes in tumor volume and were requested every 6 months for the first two years, annually for the next two years, and then once every two years for the remainder of the follow-up period. Five caliper measurements of each tumor were taken according to previously described methods19 and the measurements were compared to previous and preoperative imaging to determine changes in size. Growth or regression were defined as a 2 mm change in size in any one dimension or a 1 mm change in size in any two dimensions of the tumor. Tumors that showed transient expansion and later regression were coded as regressed. Hydrocephalus or any changes around the tumor, if present, were identified at every imaging evaluation as well. Clinical and imaging evaluations were performed at our center for the patients living in the Pittsburgh region and by the referring physicians for patients who lived far away. Each clinical evaluation involved detailed testing that included testing of cranial nerve function and evaluation of the peripheral and central nervous system.
Hearing preservation, facial neuropathy, and trigeminal neuropathy were also examined for this study. Hearing preservation was assessed in patients with testable hearing prior to radiosurgery and was determined by audiologic evaluation performed in 6 to 12 month intervals. The Gardner-Robertson hearing scale was used to classify hearing based on speech discrimination scores (SDS) and pure tone average (PTA). Serviceable hearing was defined as Gardner-Robertson grade 1 or grade 2 hearing (SDS > 50% and PTA <50 dB). Hearing was assessed prior to radiosurgery as follows: 9 patients had grade 1 hearing, 9 patients had grade 2 hearing, 22 patients had grade 3 hearing, 4 patients had grade 4 hearing, and 34 patients had grade 5 hearing. Patients with no testable hearing (grade 5) were excluded from hearing preservation analyses. Follow-up endpoints for hearing preservation were maintenance of same class hearing, serviceable hearing, or any testable hearing.
Facial neuropathy was defined as any temporary or permanent decline in facial nerve function, as measured by the House-Brackmann grading scale. Prior to radiosurgery, facial nerve function was normal (House-Brackmann grade 1) in 57 patients, grade 2 in 8 patients, grade 3 in 5 patients, grade 4 in 3 patients, grade 5 in 2 patients, and grade 6 (no facial nerve function) in 3 patients. Trigeminal neuropathy was defined as any temporary or permanent decline in trigeminal nerve function, subjectively noticed by the patient or objectively measured by the examining physician. We did not record data on corneal anesthesia.
Clinical follow-up for this series varied from 4 to 292 months (median, 66 months). Twelve (15%) patients had a clinical follow-up greater than 20 years. Imaging follow-up ranged from 4 to 276 months (median, 91 months), and 5 (6%) patients had an imaging follow-up greater than 20 years. These five patients also had a clinical follow-up greater than 20 years. Sixty-eight and 73 patients were lost to clinical and imaging follow-up, respectively. Sixteen of these patients were lost because they died within their follow-up period (within 3 years of their latest clinical or imaging follow-up date). The remainder of the patients were lost to follow-up through loss of contact, moving, and financial and insurance issues. Multiple attempts to contact patients lost to follow-up were made by phone to inquire about their clinical and imaging status.
All data was evaluated by neurosurgeons and radiation oncologists and then entered into a prospective registry as it was obtained. All study tests were validated for their utility. For statistical analysis, we constructed Kaplan-Meier plots for tumor freedom from progression and freedom from neurological deficit using the dates of first SRS, follow-up MRIs, and death or last follow-up. Standard statistical processing software (SPSS, version 17.0, SPSS Inc., Chicago, IL) was used. This study did not include a power calculation. We did not use a level of statistical significance. Data Share 1: Characteristics of 78 Patients with Vestibular Schwannomas Before Radiosurgery| # of patients (Count) | age - median years | sex-female (Percentage) | sex-male (Percentage) | prior resection (Percentage) | prior radiotherapy (Percentage) | Gardner-Robertson score-1 (Count) | Gardner-Robertson score-2 (Count) | Gardner-Robertson score-3 (Count) | Gardner-Robertson score-4 (Count) | Gardner-Robertson score-5 (Count) | House-Brackmann score-I (Count) | House-Brackmann score-II (Count) | House-Brackmann score-III (Count) | House-Brackmann score-IV (Count) | House-Brackmann score-V (Count) | House-Brackmann score-VI (Count) |
|---|
| 78 | 58.5 | 58 | 42 | 21 | 0 | 9 | 9 | 22 | 4 | 34 | 57 | 8 | 5 | 3 | 2 | 3 |
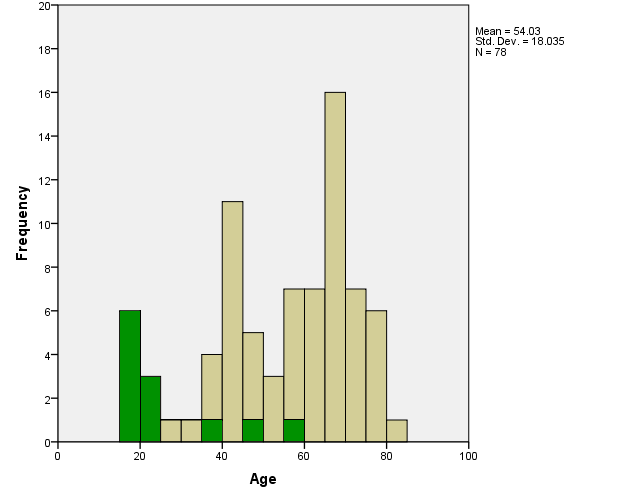 |
| Fig. 1. Histogram of the ages of patients at the time of Gamma Knife radiosurgery for their vestibular schwannoma. Green area represents a patient with neurofibromatosis type II. |
The median survival for the entire group of patients was 249 months (20.8 years).(DS. -1)
Among the 44 (56%) patients who died, median survival was 156 months.
Tumor response to radiosurgery was favorable in most of the
patients, with tumors remaining stable, regressing, and enlarging in 19 (24%),
54 (69%), and 5 (6%) of patients, respectively (Figure 2). Of the 5 enlarging
tumors, one was in a patient diagnosed with NF2, one was later diagnosed as a malignant neurofibrosarcoma after SRS,
and another developed a post-radiosurgery cyst after having also undergone a subtotal resection prior to radiosurgery. All five of the patients with
enlarging tumors underwent resection of their tumor at a mean of 35 months after radiosurgery. Image defined growth was seen 2, 5, 34, 35, and 72 months after radiosurgery. The patient with tumor growth within 2 months after radiosurgery developed brainstem compression and House-Brackmann grade 4 facial nerve function 1 week after resection of the tumor. The tumor that developed a cyst at 34 months was stereotactically drained followed by surgical resection, and the patient underwent a second gamma knife radiosurgery procedure for the remnant. The tumor that progressed 72 months after radiosurgery was diagnosed as a triton tumor after resection and has been published previously. The 20-year
actuarial rate for tumor freedom from progression was 92% ± 3.5% (rate ± standard error) (Figure 3).
Four patients developed new tumors following radiosurgery at locations unrelated to the initial radiosurgical site. Two pituitary adenomas developed in two patients 119 months and 124 months after radiosurgery. Both patients had a history of recurrent pituitary adenoma despite resection, and both adenomas were surgically resected. Two patients developed meningiomas 74 months and 143 months after radiosurgery. The first meningioma developed on the left petrous ridge in an NF2 patient treated for a right vestibular schwannoma. The second meningioma developed at the right parietal parasagittal area in a patient managed for a right vestibular schwannoma. These patients had previously been surveyed regarding outcomes and satisfaction during a 5-10 year review but we did not repeat that survey in this longer analysis.15 (Fig. -1), (Fig. -1) Patients who did not have any hearing prior to SRS (n = 34) were
not included in this hearing analysis. In the remaining 44 patients,
Gardner-Robertson hearing class was preserved in 13 (30%) patients and any
hearing, defined as Gardner-Robertson classes 1-4, was preserved in 21 (48%) of
patients.(Fig. -1), (Fig. -1) The 20-year actuarial rates of preservation of any hearing in patients with tumor volumes <1 cm3 was 54% ± 15.4% (n=12); between 1 and 3 cm3 was 62.3% ± 15% (n=11); and >3 cm3 was 29.1% ± 10.6% (n=21). The 20-year actuarial rates of preservation of same class hearing in patients with tumor volumes <1 cm3 was 33.3% ± 15.1% (n=12); between 1 and 3 cm3 was 53% ± 15.5% (n=11); and >3 cm3 was 16.3% ± 8.4% (n=21).
Serviceable hearing, defined as Gardner-Robertson classes 1 and
2, was preserved in 8 (44%) out of 18 patients. The 20-year actuarial rates of preservation of serviceable hearing in patients with tumor volumes <1 cm3 was 100% (n=3); between 1 and 3 cm3 was 66.7% ± 19.2% (n=6); and >3 cm3 was 11.1% ± 10.5% (n=9). All of the 3 patients with tumor volumes <1 cm3 maintained serviceable hearing.
20-year actuarial hearing preservation rates over all tumor volumes for same class hearing, serviceable hearing, and any testable hearing were 30% ± 7.3%, 43% ± 12%, and 44% ± 7.9%, respectively (Figures 4, 5, and 6).
Hearing class improved in 3 patients between 3 and 28 months after
treatment. The improvement in one patient from class 4 to class 3 was
temporary, lasting only 6 months after which the patient became class 5.(Fig. -1)
Facial neuropathy was defined as any temporary or permanent
decrease in facial nerve function as defined by an increase in House-Brackmann
grade. Three patients had HB grade 6 facial function prior to radiosurgery.
(Fig. -1) Twenty-nine patients developed facial nerve neuropathy between 3 days and 72
months after SRS. Normal facial nerve function was preserved in 40
of 75 patients who could be evaluated and in 39 of 59 patients with normal
facial nerve function before radiosurgery. The 20-year actuarial rate of freedom from new facial neuropathy, excluding patients with House-Brackmann grade 6 facial neuropathy prior to radiosurgery, was 59% ± 6% (Figure 7). In patients with tumor volumes <1 cm3 the rate was 76.6% ± 11.9% (n=14); between 1 and 3 cm3 was 74.4% ± 9% (n=24); and >3 cm3 was 45.6% ± 8.7% (n=40).
Trigeminal neuropathy was defined as any temporary or permanent
decrease in facial sensation, measured objectively by the physician or
subjectively by the patient during patient interview or examination. (Fig. -1) Twenty-four patients had a trigeminal nerve deficit prior to radiosurgery. Thirty-three patients developed a new or worsened trigeminal nerve deficit between 2 and 63
months of radiosurgery for a 20-year actuarial rate of 54.6%
± 6% for freedom from new trigeminal nerve deficit (Figure 8). In patients with tumor volumes <1 cm3 the rate was 90% ± 9.5% (n=14); between 1 and 3 cm3 was 57.2% ± 10.3% (n=24); and >3 cm3 was 42.4% ± 8.2% (n=40). Two patients experienced trigeminal neuralgia during their follow-up. The first patient had pain prior to radiosurgery that is still
intermittently present. The second patient developed trigeminal neuralgia 3 months after radiosurgery, which subsequently resolved 32 months later.
Radiation related injury may have developed in one NF2 patient with bilateral schwannomas and a fifth nerve tumor ipsilateral to the radiosurgically treated vestibular schwannoma. Imaging performed 3 months after radiosurgery showed typical central tumor necrosis and the patient subsequently developed a trigeminal deficit 5 months after radiosurgery. The patient was lost to follow-up shortly after, and so it is uncertain if the deficit was temporary or permanent. No comparative statistics were performed. No comparative statistics were performed. Data Share 2: Long-term Outcomes after Radiosurgery| # of patients (Count) | Follow-up time (months) | Tumor response-smaller (Count) | Tumor response-unchanged (Count) | Tumor response-larger (Count) | Hospital stay (days) | Procedures-craniotomy (Count) |
|---|
| 78 | 156 | 54 | 19 | 5 | 1 | 5 |
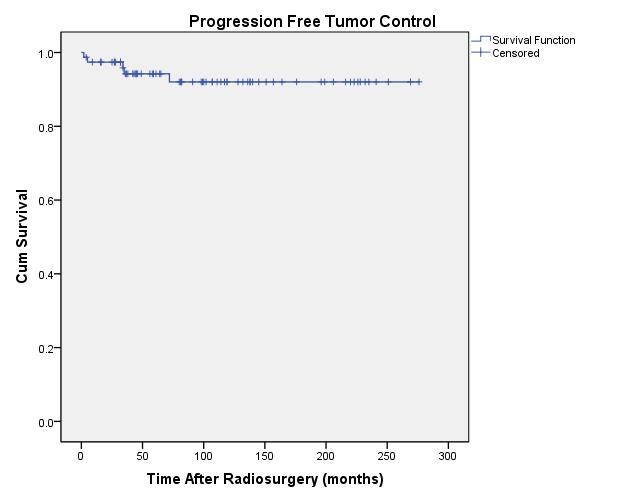 |
| Fig. 3. Actuarial plot of progression free tumor control in 77 previously untreated patients after Gamma Knife radiosurgery. |
Figure 4: Preserved Same Class Hearing
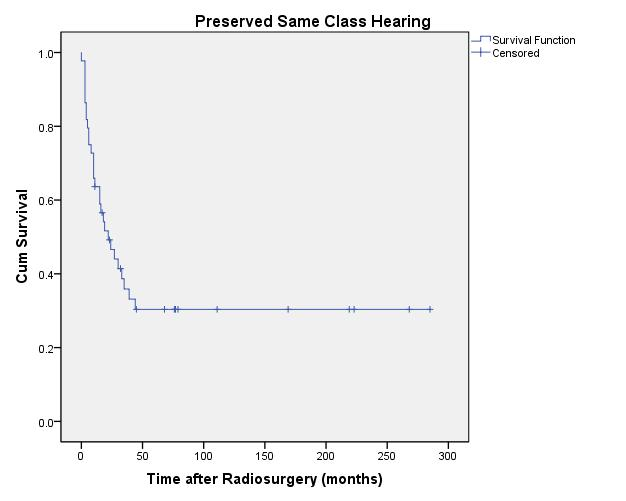 |
| Fig. 4. Actuarial plot of same Gardner-Robertson hearing level preservation of 44 previously untreated vestibular schwannoma patients (with preoperative Class 1-4 hearing) after Gamma Knife radiosurgery. |
Figure 5: Preserved Serviceable Hearing
 |
| Fig. 5. Actuarial plot of serviceable hearing preservation, defined as Gardner-Robertson Class 1 and 2, of 18 previously untreated vestibular schwannoma patients after Gamma Knife radiosurgery. |
Figure 6: Preservation of Any Testable Hearing
 |
| Fig. 6. Actuarial plot of preservation of any testable hearing, defined as Gardner-Robertson Class 1-4, of 44 previously untreated vestibular schwannoma patients after Gamma Knife radiosurgery. |
Figure 7: Freedom From New Facial Neuropathy
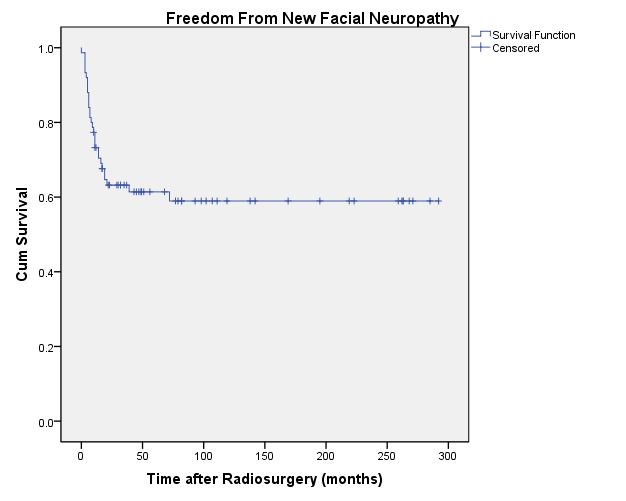 |
| Fig. 7. Actuarial plot of freedom from new facial neuropathy in 75 previously untreated vestibular schwannoma patients after Gamma Knife radiosurgery. Patients with House-Brackmann grade 6 facial neuropathy prior to radiosurgery were excluded. |
Figure 8: Freedom From New Trigeminal Neuropathy
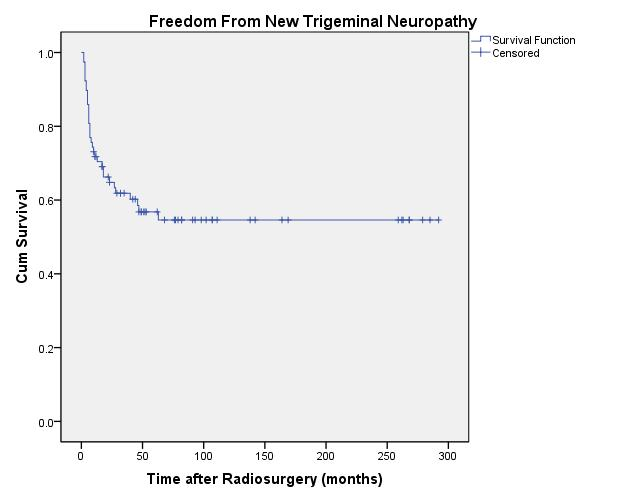 |
| Fig. 8. Actuarial plot of freedom from new trigeminal neuropathy in 78 previously untreated vestibular schwannoma patients after Gamma Knife radiosurgery. |
Over the last 25 years, stereotactic radiosurgery has become one of the most important therapeutic options for vestibular schwannoma patients. Nevertheless, the majority of reports provide outcomes over the first few years following the procedure. Such data can be particularly important in an analysis of morbidity but not in terms of long-term tumor response. This study found similar outcomes between late (10-23 years) and earlier (5-10 years) reports for the same subset of subjects. Data early in our experience showed that 55% of tumors regressed and 42% of tumors remained stable in patients with at least 1 year of follow-up, for a total imaging-defined tumor control rate of 97%.15 Foote et. al. reported an imaging-defined tumor control rate of 100% for small and stable tumors.8 Norén found a tumor control rate of 96.7%.28 Another study, where marginal tumor doses were lowered to an average of 13 Gy, showed a similar tumor control rate defined by freedom from resection of 97% at 5 years post-radiosurgery.7 Our experience at 10 years was also similar to our early experience as the resection free tumor control rate was 98% and the imaging-defined tumor control rate was 94%.18 Hearing preservation has improved as margin doses have been reduced from 18-20 Gy prior to 1992 to the more current 12-13 Gy. Early studies of patients irradiated using a median margin dose of 16-20 Gy or 14-20 Gy reported a 2-year actuarial serviceable hearing preservation rate of 41% and 46%, respectively.7,8More recent studies, where median margin doses were 13 Gy, have reported serviceable hearing rates of 73%.7 In comparison, hearing preservation after resection are typically lower but vary from 30% to 80%.23 Trigeminal and facial neuropathies typically occur within the first few years of radiosurgery. Early in our experience we reported rates of new facial and trigeminal neuropathies as 30% and 29%, respectively.7 Lower rates have been documented at a less then 5% risk of new facial or trigeminal neuropathy when margin doses have been decreased to 13 Gy.2,3,7,34
It is important to note that the approach and results documented in this report do not represent current methods. Indeed, the use of CT-based targeting was replaced with MRI in the early 1990s, and improved outcomes followed. Much has evolved in technology, imaging, and dose planning over the past 20 years. The data from this report suggests that long-term tumor control from 10 to 23 years was similar to early control. Tumor freedom from progression stabilizes 5 years after radiosurgery, suggesting that tumors that are destined to fail will resume their natural tendency to grow within a few years of being irradiated. Once this 5 year point has passed, it is unlikely a dormant tumor will begin to grow again.
Cranial nerve preservation rates were lower than in more recent reports after 1995 because this study involved patients managed early in our experience when the patients were older, the tumor margin dose averaged 18 Gy compared to the cranial nerve sparing doses of 12-13 Gy used today, and target definition was mainly with CT rather than MRI. However, our data still shows that similar to tumor size, changes in hearing occur within the first few years of radiosurgery and stabilize thereafter. Rates of preservation of same class, serviceable, and testable hearing beyond 10 years are similar to rates between 5 and 10 years. Facial and trigeminal nerve preservation followed a similar pattern. Much has changed in radiosurgery technique since these patients were cared for before 1992. Beginning around 1994, numerous aspects evolved. As noted above, targeting and dose planning included MR imaging. Margin doses were reduced to the current range of 12-13 Gy. Radiosurgical dose planning with the gamma knife utilized images integrated into the dose planning software (GammaPlan, Elekta). It became more common to offer radiosurgery to younger patients, and subsequent studies have shown that younger patients have higher hearing preservation rates.14,22 Studies in the last decade indicated the importance of cochlear dose for hearing preservation.14 Recently, the Perfexion gamma knife allowed more efficient use of beam blocking if desired for individual patients. We can conclude that the long-term data suggests that tumor progression and neurological deficits stabilize past five years in the long-term for patients with margin doses between 18 and 20 Gy. We remain vigilant with continuing follow-up assessments so that we can report the outcomes past twenty years in patients managed with current tumor margin doses, high resolution imaging-based planning, and careful attention to regional structures such as the cochlea. The results of this study do not represent what can be achieved with current techniques, but serve as a historical foundation for work in this field. The Author(s) wish to thank:Project Roles:
1. Bateman, N.,Nikolopoulos, TP.,Robinson, K.,O'Donoghue, GM., Impairments, disabilities, and handicaps after acoustic neuroma surgery. Clin. Otolaryngol 25: 62 - 65, 20002. Chopra, R.,Kondziolka, D.,Niranjan, A.,Lunsford, LD.,Flickinger, JC., Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int. J. Radiation Oncology Biol. Phys. 68: 845 - 851, 20073. Chung, WY.,Liu, KD.,Shiau, CY.,Wu, HM.,Wang, LW.,Guo, WY., et al: Gamma knife surgery for vestibular schwannoma: 10 year experience of 195 cases. J Neurosurg (Suppl) 102: 87 - 96, 20054. Delbrouck, C.,Hassid, S.,Massager, N., et al: Preservation of hearing in vestibular schwannomas treated by radiosurgery using Leksell Gamma Knife: preliminary report of a prospective Belgian clinical study. Acta oto-rhino-laryngologica Belgica 57: 197 - 204, 20035. Delsanti, C..,Roche, P.H..,Thomassin, J.M..,Regis, J.., Morphological changes of vestibular schwannomas after radiosurgical treatment: pitfalls and diagnosis of failure. Prog Neurol Surg 21: 93 - 97, 20086. Flickinger, JC.,Kondziolka, D.,Niranjan, A.,Lunsford, LD., Results of acoustic neuroma radiosurgery: an analysis of 5 years' experience using current methods. J Neurosurg 94: 1 - 6, 20017. Flickinger, JC.,Lunsford, LD.,Coffey, RJ.,Linskey, ME.,Bissonette, DJ.,Maitz, AH., et al: Radiosurgery of acoustic neuromas. Cancer 67: 345 - 353, 19918. Foote, RL.,Coffey, RJ.,Swanson, JW.,Harner, SG.,Beatty, CW.,Kline, RW., et al: Stereotactic radiosurgery using the gamma knife for acoustic neuromas. Int. J. Radiation Oncology Biol. Phys. 32: 1153 - 1160, 19959. Gormley, WB.,Sekhar, LN.,Wright, DC.,Kamerer, D.,Schessel, D., Acoustic neuromas: results of current surgical management. Neurosurgery 41: 50 - 60, 199710. Hasegawa, T.,Kida, Y.,Kobayashi, T.,Yoshimoto, M.,Mori, Y.,Yoshida, J., Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: 10-year follow up. J Neurosurg 102: 10 - 16, 200511. Hirsch, A.,Noren, G., Audiological findings after stereotactic radiosurgery in acoustic neurinomas.. Acta Otolaryngol 106(3-4): 244 - 251, 198812. Horstmann, GA.,Albertus, VE., Gamma knife model C with the automatic positioning system and its impact on the treatment of vestibular schwannomas. J Neurosurg 97: 450 - 455, 200213. Inoue, HK., Low-dose radiosurgery for large vestibular schwannomas: long-term results of functional preservation. J Neurosurg 102: 111 - 113, 200514. Kano, H.,Kondziolka, D.,Khan, A.,Flickinger, JC.,Lunsford, LD., Predictors of hearing preservation after stereotactic radiosurgery for acoustic neuroma. J Neurosurg 111 (4): 863 - 873, 200915. Kondziolka, D.,Lunsford, LD.,McLaughlin, MR.,Flickinger, JC., Long-term outcomes after radiosurgery for acoustic neuromas.. N Engl J Med 339(20): 1426 - 1433, 199816. Kondziolka, D.,Lunsford, LD.,Flickinger, JC., Acoustic neuroma radiosurgery. Origins, contemporary use and future expectations.. Neuro-Chirurgie 50: 427 - 435, 200417. Kondziolka, D.,Lunsford, LD.,Flickinger, JC., Gamma knife radiosurgery for vestibular schwannomas.. Neurosurg Clin N Am 11: 651 - 658, 200018. Kondziolka, D.,Nathoo, N.,Flickinger, JC.,Niranjan, A.,Maitz, AH.,Lunsford, LD., Long-term results after radiosurgery for benign intracranial tumors. Neurosurgery 53: 815 - 821, 200319. Linskey, ME.,Lunsford, LD.,Flickinger, JC., Neuroimaging of acoustic nerve sheath tumors after stereotaxic radiosurgery.. AJNR Am J Neuroradiol 12(6): 1165 - 1175, 199120. Linskey, ME., Stereotactic radiosurgery versus stereotactic radiotherapy for patients with vestibular schwannoma: a Leksell Gamma Knife Society 2000 debate. J Neurosurg 93: 90 - 95, 200021. Linskey, ME.,Lunsford, LD.,Flickinger, JC., Tumor control after stereotactic radiosurgery in neurofibromatosis patients with bilateral acoustic tumors.. Neurosurgery 31: 829 - 838, 199222. Lobato-Polo, J.,Kondziolka, D.,Zorro, O.,Kano, H.,Flickinger, JC.,Lunsford, LD., Gamma knife radiosurgery in younger patients with vestibular schwannomas. Neurosurgery 65 (2): 294 - 300, 200923. Lunsford, LD.,Niranjan, A.,Flickinger, JC.,Kondziolka, D., Navigating change and the acoustic neuroma story: methods, outcomes, and myths. Clinical Neurosurgery 55: 47 - 61, 200824. Lunsford, LD.,Niranjan, A.,Flickinger, JC.,Maitz, A.,Kondziolka, D., Radiosurgery of vestibular schwannoms: summary of experience in 829 cases. J Neurosurg (Suppl) 102: 195 - 199, 200525. Mazzoni, A.,Calabrese, V.,Moschini, L., Residual and recurrent acoustic neuroma in hearing preservation procedures: neuroradiologic and surgical findings. Skull Base Surgery 6: 105 - 112, 199626. Myrseth, E.,Møller, P.,Pedersen, PH.,Vassbotn, FS.,Wentzel-Larsen, T.,Lund-Johansen, M., Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife raidosurgery. Neurosurgery 56: 927 - 935, 200527. Niranjan, A.,Lunsford, LD.,Flickinger, JC.,Maitz, A.,Kondziolka, D., Dose reduction improves hearing preservation rates after intracanalicular acoustic tumor radiosurgery. Neurosurgery 45: 753 - 762, 199928. Noren, G.,Arndt, J.,Hindmarsh, T., Stereotactic radiosurgery in cases of acoustic neurinoma: further experiences.. Neurosurgery 13(1): 12 - 22, 198329. Pollock, BE.,Driscoll, CL.,Foote, RL.,Link, MJ.,Gordman, DA.,Bauch, CD., Patient outcomes after vestibular schwannoma management: a prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery 59: 77 - 85, 200630. Regis, J.,Pellet, W.,Delsanti, C.,Dufour, H.,Hughes Roche, P.,Marc Thomassin, J., et al: Functional outcome after gamma knife surgery or microsurgery for vestibular schwannomas. J Neurosurg 97: 1091 - 1100, 200231. Samii, M.,Matthines, C., Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery 40: 11 - 23, 199732. Sampath, P.,Holliday, MJ.,Brem, H.,Niparko, JK.,Long, DM., Facial nerve injury in acoustic neuroma (vestibular schwannoma) surgery: etiology and prevention. J Neurosurg 87: 60 - 66, 199733. Sughrue, ME.,Kaur, R.,Rutkowski, MJ.,Kane, AJ.,Kaur, G.,Yang, I., et al: Extent of resection and the long-term durability of vestibular schwannoma surgery. J Neurosurg 114: 1218 - 1223, 201134. Unger, F.,Walch, C.,Schrottner, O.,Eustacchio, S.,Sutter, B.,Pendl, G., Cranial nerve preservation after radiosurgery of vestibular schwannomas.. Acta Neurochir Suppl 84: 77 - 83, 2002
|

